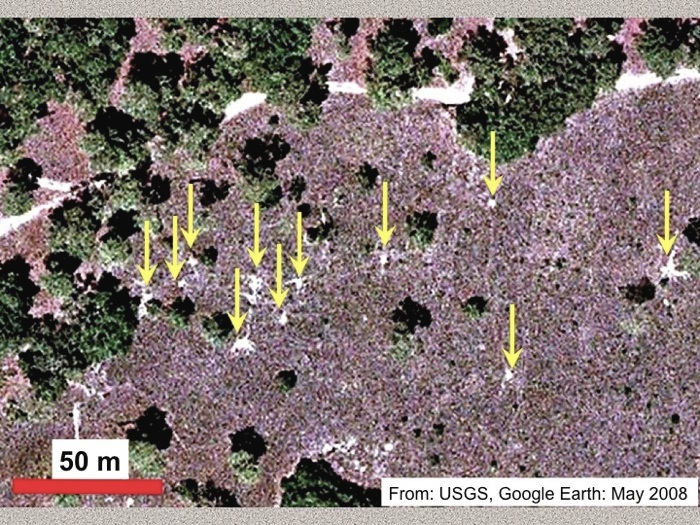
Two weeks ago, during an all-too-brief visit to Jekyll Island (Georgia) over the Thanksgiving holiday weekend, I decided to check in on some old friends. When I was first introduced to them about four years ago (2008), their presence on Jekyll was a big surprise for me. But thanks to their distinctive traces and a little bit of detective work, I now know they’re on other Georgia barrier islands, too.
 Why look, miniature volcanoes in the middle of a maritime forest on Jekyll Island! Or, could they be something else? (In science, that’s what we like to call an “alternative hypothesis.”) Photo scale (left) in centimeters. (Photograph by Anthony Martin.)
Why look, miniature volcanoes in the middle of a maritime forest on Jekyll Island! Or, could they be something else? (In science, that’s what we like to call an “alternative hypothesis.”) Photo scale (left) in centimeters. (Photograph by Anthony Martin.)
These “friends” were conical towers, which look like small lumpy volcanoes (stratovolcanoes, that is, not shield volcanoes), were the traces of freshwater crayfish. A few of the structures, composed of piled balls of sandy mud, also had circular holes in their centers, and they had all seemingly popped out of the forest floor along the edge of a pool of fresh water. All I needed to do to find them was look in the same place where I was first introduced to them, which was by a Jekyll Island resident who knew about their whereabouts.
The towers were 10-25 cm (4-6 in) wide at their bases, 7-10 cm (3-4 in) tall, and each of the rounded, oval balls of sediment was about 1-1.5 cm (0.4-0.6 in) wide. The overall appearance of the towers said “still fresh,” having not been appreciably weathered, and all that I saw in the area looked about the same age. Knowing a little bit about crayfish behavior, I figure they were made just after the last rainfall on Jekyll, maybe a week or so before I spotted them.
 Close-up of a crayfish tower, with a circular hole in the center (that’s the burrow). Scale in centimeters. (Photograph by Anthony Martin, taken on Jekyll Island, Georgia.)
Close-up of a crayfish tower, with a circular hole in the center (that’s the burrow). Scale in centimeters. (Photograph by Anthony Martin, taken on Jekyll Island, Georgia.)
Crayfish that dig burrows adjust their depth according to the water table, which they must do to stay alive because they have gills. If the water table drops, they burrow deeper, but if the water table rises, they move their burrows up. For example, where I live here in the metro Atlanta area, crayfish towers often pop up in people’s backyards the day after a hard rain. (This also means that these people need to get flood insurance, because their backyards are on a floodplain. Thus also demonstrating yet another practical reason to know a little basic ichnology.)
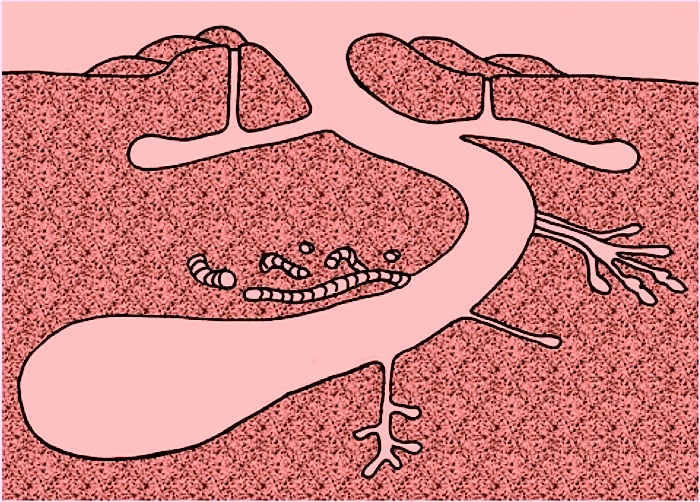
Burrowing was (and still is) accomplished by crayfish using their prominent claws (chelipeds) as spades, rolling up the balls of sediment and placing them outside of the burrow entrance, and thus building up towers. But they also smooth out burrow interiors with their bodies through up-and-down and back-and-forth movement, resulting in their burrows having near-perfect circular cross sections. Crayfish burrow systems can be complicated, with vertical shafts connecting the surface with the below-ground parts, which can consist of branching horizontal tunnels and chambers; the last of these can even be occupied by multiple crayfish.
When I first saw these these towers and burrow cross-sections on Jekyll Island in 2008, I immediately knew they were from crayfish. My certainty was because such traces had been described in loving detail by crayfish researchers and ichnologists, linking these directly to their crustacean makers. In fact, just a few months ago, I saw an example of this connection between traces and tracemakers in my home of Decatur, Georgia, where the drying of a human-made pond there caused the crayfish to burrow into the former pond bottom and move about on its sediments in a desperate attempt to stay wet.
 A high density of crayfish burrows in a recently drained human-made pond in Decatur, Georgia. Note the similarity of the towers, circular burrow cross-sections, and rounded balls of sediment to those of the Jekyll Island crayfish burrows. Scale with centimeters. (Photograph by Anthony Martin.)
A high density of crayfish burrows in a recently drained human-made pond in Decatur, Georgia. Note the similarity of the towers, circular burrow cross-sections, and rounded balls of sediment to those of the Jekyll Island crayfish burrows. Scale with centimeters. (Photograph by Anthony Martin.)
 “Are you looking at me?” Crayfish, about 5 cm (2 in) across, and probably a species of Procambarus, copping an attitude while guarding its burrow entrance. (Photograph by Anthony Martin, taken in Decatur, Georgia.)
“Are you looking at me?” Crayfish, about 5 cm (2 in) across, and probably a species of Procambarus, copping an attitude while guarding its burrow entrance. (Photograph by Anthony Martin, taken in Decatur, Georgia.)
With about 70 species documented in the state, Georgia is quite rich in crayfish diversity, qualifying it and bordering states in the southeastern U.S as a “biodiversity hotspot” for these animals. Freshwater crayfish are also geographically widespread – occurring in North and South America, Europe, Madagascar, Australia, New Zealand, New Guinea – a direct result of plate tectonics, which spread and isolated populations from one another during their evolutionary history.
In terms of that history, these crustaceans (decapods, more specifically) split from a common ancestor with marine lobsters about 240 million years ago, an age based on molecular clocks, which have been integrated with fossil evidence. I’ve also seen trace fossils that look very much like crayfish burrows in Late Triassic rocks, from about 210 million years ago, which suggests that burrowing began in this lineage early in the Mesozoic Era.
In a 2008 article I co-authored and published with six other scientists – three paleontologists and three zoologists – we described fossil burrows in rocks from the Early Cretaceous Period (about 115-105 million years ago) of Australia, and named what is still the oldest fossil crayfish in the Southern Hemisphere, Palaeoechinastacus australanus. In this article, we pointed out how burrowing was an adaptation that likely helped these crayfish survive polar winters in Australia during the Cretaceous, but also how burrowing abilities in general have given crayfish an upper claw, er, hand in making it past environmental crises in the geologic past.
 Here’s the oldest known fossil freshwater crayfish in Australia and the rest of the Southern Hemisphere, Palaeoechinastacus australanus (= “ancient spiny crayfish of Australia”), found in 105-million-year-old rocks (Early Cretaceous) of southern Victoria. Not everything is there, but you can see most of its tail to the left and the right-side legs. Specimen is Museum Victoria, Melbourne, Australia. (Photograph by Anthony Martin.)
Here’s the oldest known fossil freshwater crayfish in Australia and the rest of the Southern Hemisphere, Palaeoechinastacus australanus (= “ancient spiny crayfish of Australia”), found in 105-million-year-old rocks (Early Cretaceous) of southern Victoria. Not everything is there, but you can see most of its tail to the left and the right-side legs. Specimen is Museum Victoria, Melbourne, Australia. (Photograph by Anthony Martin.)
 And here’s a bedding plane (horizontal) view of trace fossils attributed to freshwater crayfish burrows, preserved in 115-million-year-old rocks (also Early Cretaceous) near Inverloch, Victoria (Australia). The burrows were filled with sand originally, which cemented differently from the surrounding sediment, making them stand out in positive relief as they weather on the outcrop. Scale = 10 cm (4 in). (Photograph by Anthony Martin.)
And here’s a bedding plane (horizontal) view of trace fossils attributed to freshwater crayfish burrows, preserved in 115-million-year-old rocks (also Early Cretaceous) near Inverloch, Victoria (Australia). The burrows were filled with sand originally, which cemented differently from the surrounding sediment, making them stand out in positive relief as they weather on the outcrop. Scale = 10 cm (4 in). (Photograph by Anthony Martin.)
So how did these crayfish get onto the Georgia barrier islands? Before answering that, I can tell you how they did not get there, which was from people. Because these are burrowing (infaunal) crayfish, and not ones that hang out on lake or stream bottoms (also known as epibenthic), it’s not very likely that humans purposefully introduced them on the islands for aquaculture. Let’s just say that digging up each crayfish burrow, which may or may not contain a crayfish, would require too much work to make crayfish etoufee worth the effort, no matter how good your recipe might be.
 Mmmmm, flavorful freshwater decapod concoction [drooling sounds]. But first imagine having to dig up every single crayfish for this dish. Just to prevent this from happening, your recipe should have some qualifying statement, such as, “Make sure to use epibenthic crayfish, not infaunal ones!” (Original image, modified slightly by me, from Wikipedia Commons here.)
Mmmmm, flavorful freshwater decapod concoction [drooling sounds]. But first imagine having to dig up every single crayfish for this dish. Just to prevent this from happening, your recipe should have some qualifying statement, such as, “Make sure to use epibenthic crayfish, not infaunal ones!” (Original image, modified slightly by me, from Wikipedia Commons here.)
Another point to remember about crayfish is that they are freshwater-only animals, incapable of tolerating salt-water immersion, let alone swimming kilometers through marine-flavored waters to reach offshore islands. Yet I’ve seen their traces on Jekyll and two other Georgia barrier islands, and crayfish species have been reported from two additional islands. (For now I won’t say which other islands or identify the probable species of these crayfish until they’ve been properly studied. Sorry.)
What might seem strange to most people, though, is that I still haven’t seen a single living crayfish on any of the Georgia barrier islands. Nonetheless, seeing and documenting their traces is good enough for me to know where they’re living and how they’re behaving. This again demonstrates one of the many advantages of ichnology: you don’t actually have to see an animal to know it’s there, just as long as it leaves lots of identifiable traces.
Oh yeah: almost forgot about the title of this post. What’s my explanation for how the crayfish got to the islands, including Jekyll? I think they lived on the islands before they were islands. In other words, present-day crayfish on the islands descended from ones that originally lived on the mainland part of Georgia, but these were cut off from their original homeland by the last major sea-level rise (well before the current one, that is). This rise started as long as 11,000 years ago, when the last great ice age of the Pleistocene ended, shedding water from continental glaciers and expanding the seas.
So think of a salty moat filling in the low areas between what are now the Georgia barrier islands and the rest of Georgia, with crayfish on either side of it, metaphorically waving goodbye to one another with their claws. In this scenario, the crayfish of the Georgia barrier islands may represent relics left behind and isolated from their ancestral populations. They may have even undergone genetic drift and became new species, or are well on their way to reproductive isolation from their mainland relatives. But that’s just speculation on my part right now. Like I said, these critters need to be studied before anything can be said about them.
All of this neatly illustrates how our knowledge of the geological past ties in with the present, as well as how ichnology can be applied to conservation biology. With regard to the latter, these little muddy crayfish towers exemplify one of the dangers associated with any rapid, careless development of the Georgia barrier islands. What if most people aren’t aware of the unique plants and animals on the islands because at least some of this biodiversity lies below their feet? Without such knowledge, unheeded development may very well wipe out rare or previously unknown species that have been part of the ecological legacy of the Georgia coast for the past 10,000 years.
This is one of many reasons why environmental protection of the islands is still needed, even on semi-developed one like Jekyll. Fortunately, motivated people are working toward such protection on Jekyll, and most other Georgia barrier islands are under some sort of state or federal protection, or privately owned as preserves.
 Nice maritime forest you got there. It’d be a shame if something happened to it. (Photograph by Anthony Martin, taken on Jekyll Island.)
Nice maritime forest you got there. It’d be a shame if something happened to it. (Photograph by Anthony Martin, taken on Jekyll Island.)
What’s also happened on Jekyll Island is increased ecotourism, highlighted by the success of the Georgia Sea Turtle Center. The center, which opened in 2007, has a rehabilitation center for injured turtles, educates the public about sea turtles nesting on the Georgia coast, and helps to monitor turtle nests on Jekyll during the nesting season. And just how is this monitoring done? By looking for tracks of the nesting mothers on the beaches of Jekyll during nesting season, of course. (Say, didn’t I say something previously about using ichnology in conservation biology?)
So can a Jekyll Island Crayfish Center be far behind? Um, no. Still, it’s time to start thinking of species on the Georgia barrier islands and their traces as assets, bragging points that can be used to bolster ecotourism on the coast. Barrier-island biodiversity is an economic resource that will continue to pay off as long as the species survive and their habitats are protected, while simultaneously feeding our sense of wonder at how these species, including burrowing freshwater crayfish, got to the islands in the first place.
Further Reading
Breinholt, J., Ada, M. P.-L., and Crandall, K.A. 2009. The timing of the diversification of the freshwater crayfish. In Martin, J.W., Crandall, K.A., and Felder, D.L. (editors), Decapod Crustacean Phylogenetics, CRC Press, Boca Raton, Florida: 343-355.
Hobbs, H.H., Jr. 1981. The Crayfishes of Georgia. Smithsonian Institute Press, Washington, D.C.: 549 p.
Hobbs, H.H., Jr. 1988. Crayfish distribution, adaptive radiation and evolution. In: Holdich, D.M., Lowery, R.S. (editors), Freshwater Crayfish: Biology, Management and Exploitation. Croom Helm, London: 52-82.
Martin, A.J. 2011. Ichnology in a time of climate change: predicted effects of rising sea level and temperatures on organismal traces of the Georgia coast. Geological Society of America, Abstracts with Programs, 43(2): 86. Link here.
Martin, A.J., Rich, T.H., Poore, G.C.B., Schultz, M.B., Austin, C.M., Kool, L., and Vickers-Rich, P. 2008. Fossil evidence from Australia for oldest known freshwater crayfish in Gondwana. Gondwana Research, 14: 287-296.
P.S. So you’d like to hear more details on the crayfish of the Georgia barrier islands? Well, then you’re going to have to read my book, which starts out Chapter 5 (on terrestrial invertebrate traces) with a section titled The Crayfish of Jekyll Island. Yes, that’s a sales pitch, but you can also request your public library to get it, or borrow a copy from a friend. Which makes this more of a “knowledge pitch.”