(The following is the third part of a series about traces of invasive species of mammals on the Georgia barrier islands and the ecological effects of these traces. Here is an introduction to the topic, the first entry about the feral horses of Cumberland Island, and the second entry about the feral cattle of Sapleo Island.)
Anytime I hear someone refer to a Georgia barrier island as “pristine,” I wince a little bit, smile, and say, “Well, bless your heart.” The truth is, nearly every island on the Georgia coast, no matter how beautiful, is not in a pristine state, having been considerably altered by humans over the past 4,500 years, whether these were Native Americans, Europeans, or Americans. These varying degrees of change are sometimes subtle but nonetheless there, denoted by the loss of original habitats and native species or the addition of non-native species.
Still, one Georgia barrier island comes close to fulfilling this idealistic label: Wassaw Island, which during its 1,000-year geologic history somehow escaped commercial logging, agriculture, animal husbandry, and year-round settlements. Partially because of this legacy, Wassaw is designated as a National Wildlife Refuge, and is reserved especially for ground-nesting birds. One of the ways this island works well as a refuge for these birds is – as of this writing – its “hog free” status, a condition that can be tested with each visit by looking for the obvious traces of this invasive species.
 The interior of Wassaw Island, with maritime forest surrounding a freshwater wetland created by alligators, the rightful owners of the island. On Wassaw, there are no tracks or signs of feral hogs, qualifying it as a “pristine” island. (Photograph by Anthony Martin.]
The interior of Wassaw Island, with maritime forest surrounding a freshwater wetland created by alligators, the rightful owners of the island. On Wassaw, there are no tracks or signs of feral hogs, qualifying it as a “pristine” island. (Photograph by Anthony Martin.]
 Contrast this with Cumberland Island National Seashore, where hogs run wild and freely. The huge pits here are in an intertidal zone of a beach on the northwest corner of the island. Naturalist Carol Ruckdeschel (background) for scale. (Photograph by Anthony Martin.)
Contrast this with Cumberland Island National Seashore, where hogs run wild and freely. The huge pits here are in an intertidal zone of a beach on the northwest corner of the island. Naturalist Carol Ruckdeschel (background) for scale. (Photograph by Anthony Martin.)
Feral hogs (Sus scrofa) have a special place in the rogue’s gallery of invasive mammals on the Georgia barrier islands, and most people agree they are the worst of the lot. Hogs are on every large undeveloped island – Cumberland, Sapelo, St. Catherines, and Ossabaw – and they wreak ecological havoc wherever they roam. The widespread damage they cause is largely related to their voracious and omnivorous diet, in which they seek out and eat nearly any foodstuff, whether fungal, plant, or animal, live or dead. Their fine sense of smell is their greatest asset in this respect: every time I have tracked feral hogs, their tracks show head-down-nose-to-the-ground movement as the norm, punctuated by digging that uses a combination of their snouts and front hooves to tear up the ground in their quest for food. In other words, they generally act like, well, you know what.
Most importantly from the standpoint of native animals that try to live more than one generation beyond a single hog meal, feral hogs eat eggs. Hence ground-nesting birds and turtles are among their victims, and hogs are quite keen on eating sea turtle eggs. Mothers of all three species of sea turtles that nest on the Georgia coast – loggerhead (Caretta caretta), green (Chelonia mydas), and leatherback (Dermochelys coriacea) – dig subsurface nests filled with 100-150 eggs full of protein and other nutrients, making tempting targets for any free-ranging feral hogs. Similarly, hogs also threaten another salt-water turtle, the diamondback terrapin (Malaclemys terrapin); this turtle lays its eggs in shallow nests near the edges of salt marshes, which hogs manage to find. Conservation efforts to save diamondback terrapins from human predation have mostly succeeded (it used to be a tasty ingredient in soups), but hogs can’t read and don’t discriminate when it comes to eating eggs. Here is where feral hogs are particularly dangerous as an invasive species: unlike feral horses or cattle, which “merely” degrade parts of their ecosystems: feral hogs can contribute directly to the extinction of native species. As I often tell my students, if you want to cause a species to go extinct, stop it from reproducing.
 Sea-turtle nest on Sapelo Island, marked by a stake and protected by plastic fencing to prevent feral hog and raccoon depredation of its eggs. An individual raccoon would only eat about 1/3 of the eggs in a sea-turtle nest, whereas pigs would just keep on eating. (Photograph by Anthony Martin.)
Sea-turtle nest on Sapelo Island, marked by a stake and protected by plastic fencing to prevent feral hog and raccoon depredation of its eggs. An individual raccoon would only eat about 1/3 of the eggs in a sea-turtle nest, whereas pigs would just keep on eating. (Photograph by Anthony Martin.)
As an ichnologist, though, what astounds me the most about these hogs is the extremely wide ecological range of their traces. I have seen their tracks – often made by groups traveling together – in the deepest interiors of maritime forests, in freshwater wetlands, and crossing back-dune meadows, high salt marshes, coastal dunes, and beaches. If their traces became trace fossils, paleontologists would refer to them as a facies-crossing species, in which facies (think “face”) are the identifiable traits of a sedimentary environment preserved in the geologic record. Based on their tracks and sign, they are ubiquitous in terrestrial and marginal-marine environments. Oh, and did I mention they are also good swimmers? Swimming across a tidal channel at low tide is an easy feat for them, enabling hogs to spread from island to island, without the assistance of humans.
 Run away, run away! Feral hogs in a St. Catherines Island salt marsh, consisting of two juveniles and an adult, do not stick around to see whether humans are going to shoot them; they just assume so. This sighting, along with their widespread tracks and other traces, show how feral hogs can occupy and affect nearly every environment on a Georgia barrier island. (Photograph by Anthony Martin.)
Run away, run away! Feral hogs in a St. Catherines Island salt marsh, consisting of two juveniles and an adult, do not stick around to see whether humans are going to shoot them; they just assume so. This sighting, along with their widespread tracks and other traces, show how feral hogs can occupy and affect nearly every environment on a Georgia barrier island. (Photograph by Anthony Martin.)
So to better understand why feral hogs are such successful invaders of the Georgia islands, it’s helpful to think about their evolutionary history. As expected, this history is complicated, just like that of any domesticated species in which selective breeding narrowed the genetic diversity we see today. About 15 subspecies of Sus scrofa have been identified, making its recent family tree look rather bushy. Based on genetic studies, divergence between wild species of Sus scrofa (so-called “wild boars”) and various subspecies may have happened as long ago as 500,000 years ago in Eurasia, although humans did not capture and start breeding them until about 9,000 years ago.
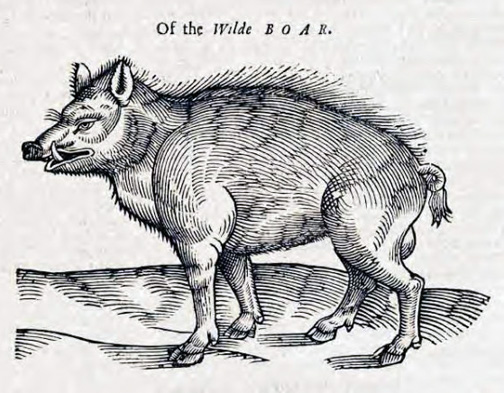 Depiction of a European wild boar from 1658, in The History of Four-Footed Beasts and Serpents by Edward Topsell. Original image from a woodcut, digital image in Wikipedia Commons here.
Depiction of a European wild boar from 1658, in The History of Four-Footed Beasts and Serpents by Edward Topsell. Original image from a woodcut, digital image in Wikipedia Commons here.
The closest extant relatives to these hogs native to North America are peccaries, which live in the southwestern U.S., Central America, and South America. However, peccaries are recent migrants to North America, and only one Pleistocene species (Mylohyus nasutus) is known from the fossil record of the eastern U.S. This means that the post-Pleistocene ecosystems of the eastern U.S., and especially those of the Georgia barrier islands, have never encountered anything like these animals. Also, unlike the feral horses of Cumberland Island and the feral cattle of Sapelo Island, the feral hogs of the Georgia barrier islands were likely introduced early in European colonization of the coast, and may have started with the Spanish in the 16th century.
Unfortunately, part of the selective breeding of Eurasian hogs was for early sexual maturity and large litter sizes. Female feral hogs can reach breeding age at 5 months, and litters typically have 4-8 piglets, but can be greater than 12; females also can produce three litters in just more than a year. Do the math, and that adds up to a lot of pigs in a short amount of time. Furthermore, on Georgia barrier islands with few year-round human residents, the only predation pressures young piglets face daily include raptors (no, not that kind of raptor) or alligators. This means young hogs reach sexual maturity soon enough to rapidly overrun a barrier island.
 Feral hog trackway in a sandy intertidal zone of Cumberland Island, showing a typical gallop pattern (four tracks together –> space –> four tracks together), symbolizing how they are running roughshod over this and other islands. (Photograph by Anthony Martin.)
Feral hog trackway in a sandy intertidal zone of Cumberland Island, showing a typical gallop pattern (four tracks together –> space –> four tracks together), symbolizing how they are running roughshod over this and other islands. (Photograph by Anthony Martin.)
Yet as we have learned in North America, and particularly on the Georgia barrier islands, feral hogs rapidly revert to their Pleistocene roots. Similar to the feral cattle of Sapelo Island, these hogs are rarely seen by people, especially on islands where humans regularly hunt them. Every time I have spotted them on Cumberland, Sapelo, St. Catherines, or Ossabaw, they instantly turn around, briefly flash their potential pork loins and ham hocks, and flee. As anyone who has raised hogs can tell you, pigs are smart and learn quickly. Hence I imagine that after only one or two shootings of their siblings or parents, they readily associate upright bipeds with imminent death, especially if these bipeds are carrying “boomsticks.” (Speaking of which, I know of at least one sea turtle researcher who does his part to decrease feral hog populations – while also feeding the local vultures – through his able use of such a baby-sea-turtle-protection device.)
Hence much of what we learn about these free-ranging pigs and their behaviors in the context of the Georgia barrier islands is from their traces. Among the most commonly encountered feral hog traces are:
• Tracks
• Rooting pits
• Wallows
• Feces
Feral hog tracks are potentially confused with deer tracks, as they both consist of paired hoofprints and overlap in their size ranges, which are about 2.5-6 cm (1-2.5 in) long. Nonetheless, feral hog tracks are less “pointed,” have nearly equal widths and lengths, rounded ends, and the two hoofs often splay. Two dew claws – vestigial toes – frequently register behind the hoofs, especially when hogs step into soft sand or mud or are running. Trackways normally show indirect register of the rear foot onto the front footprint in a diagonal walking pattern, but can also display a whole range from slow walk to full gallop patterns. With repeated use of pathways, trackways become trails, although I’m not sure if hogs are merely using and expanding previously existing whitetail deer trails, if they are blazing their own, or a combination of the two. (I suspect the last of these is the most likely.)
 Feral hog tracks, showing nearly equal lengths and widths, rounded ends, and splaying of hooves, all three of which help to distinguish these from whitetail deer tracks. Scale in centimeters. (Photo by Anthony Martin, taken on Sapelo Island.)
Feral hog tracks, showing nearly equal lengths and widths, rounded ends, and splaying of hooves, all three of which help to distinguish these from whitetail deer tracks. Scale in centimeters. (Photo by Anthony Martin, taken on Sapelo Island.)
 Feral hog trackway on upper part of a sandy beach (moving parallel to shore), showing slow diagonal walking pattern, verified by hoof dragmarks between sets of tracks. Scale = 10 cm (4 in). (Photo by Anthony Martin, taken on St. Catherines Island.)
Feral hog trackway on upper part of a sandy beach (moving parallel to shore), showing slow diagonal walking pattern, verified by hoof dragmarks between sets of tracks. Scale = 10 cm (4 in). (Photo by Anthony Martin, taken on St. Catherines Island.)
Rooting pits are broad but shallow depressions – as much as 5 m (16 ft) wide and 30 cm (1 ft) deep – that are the direct result of feral hogs digging for food. In most instances, I suspect they are going for fungi and plant roots, but they probably also eat insect larvae, lizards, small mammals, and any other animals that live in burrows. These pits are typically in maritime forests and back-dune meadows, but I have seen them in salt marshes and dunes, and, most surprisingly, in the intertidal areas of beaches. What are they seeking and eating in beach sands? I think anything dead and buried that might be giving off an odor. I have even seen their tracks associated with broken carapaces of horseshoe crabs (Limulus polyphemus), a menu item that never would have occurred to me if I had not seen these traces.
 Rooting pit in back-dune meadow on St. Catherines Island. Former student, who answers to the parent-given appellation of “Andrew,” for scale. (Photograph by Anthony Martin.)
Rooting pit in back-dune meadow on St. Catherines Island. Former student, who answers to the parent-given appellation of “Andrew,” for scale. (Photograph by Anthony Martin.)
 Evidence of feral hog feeding on a horseshoe crab (Limulus polyphemus). All I can say is, it must have been really hungry. (Photo by Anthony Martin, taken on St. Catherines Island.)
Evidence of feral hog feeding on a horseshoe crab (Limulus polyphemus). All I can say is, it must have been really hungry. (Photo by Anthony Martin, taken on St. Catherines Island.)
Wallows are similar in size and appearance to rooting pits, but have a different purpose, which is to provide hogs with relief from both the Georgia summer heat and biting insects that invariably go with this heat. These structures are often near freshwater wetlands in island interiors, but I’ve seen them next to salt marshes, too. If these wallows intersect the local water table, they also make for attractive little ponds for mosquitoes to breed, meaning these hog traces indirectly contribute to the potential spread of mosquito-borne diseases.
 Wallow in maritime forest, Sapelo Island, with a standing pool of water indicating the local water table at the time. (Photo by Anthony Martin.)
Wallow in maritime forest, Sapelo Island, with a standing pool of water indicating the local water table at the time. (Photo by Anthony Martin.)
Hog feces may look initially like deer pellets, but tend to aggregate in clusters. Most of the ones I have seen are filled with vegetation, but the extremely varied diets of feral hogs means you should expect nearly anything to show up in their scat.
 Feral hog feces on Sapelo Island, which is more clumped than that of whitetail deer. Scale in centimeters. (Photo by Anthony Martin, taken on Sapelo Island.)
Feral hog feces on Sapelo Island, which is more clumped than that of whitetail deer. Scale in centimeters. (Photo by Anthony Martin, taken on Sapelo Island.)
Which of these traces would make it into the fossil record? I would certainly bet on at least some of their tracks getting preserved, based on the sheer ubiquity of these traces in nearly every sedimentary environment of a Georgia barrier island. Other likely traces would be their pits and wallows, although their broad size and shallow depths would make them difficult to recognize unless directly associated with tracks. Feces would be the least likely to make it into the fossil record as coprolites, unless these contained a fair amount of bone or other mineralized stuff, which could happen with hogs.
What to do about these hogs, and how to decrease the impacts of their traces? Well, as most people know, pigs are wonderful, magical animals that were domesticated specifically for their versatile animal protein. So one solution is more active and year-round hunting of hogs, and using them to supplement breakfasts, lunches, and dinners of local residents on the Georgia coast, a neat blend of reducing a harmful feral species while encouraging a chic “locavore” label on such food.
However, the sheer numbers of hogs on some of the islands would likely require a more systematic slaughter to make a dent in their numbers, an approach that would probably deter any ecotourism unrelated to hog hunting. (Let’s just say that firearms and bird watching are an uneasy mix.) The introduction of native predators is another possible solution. For example, Cumberland Island has a population of bobcats (Lynx rufus) that was introduced primarily to control the whitetail deer population, but these cats probably also take a toll on the feral hogs (although how much is unknown). I have even heard suggestions of reintroducing red wolves (Canis rufus) to a few of the islands. These pack-hunting predators were native to the southeastern U.S. before their extirpation by fearful European settlers, and probably would reduce feral hog populations, but just how much of an impact they would have is hard to predict.
In summary, the feral horses, cattle, and hogs of the Georgia barrier islands have significant effects on the ecology and geology of the Georgia barrier islands, and will continue to do so until creative solutions are proposed and implemented to reduce and otherwise manage their numbers. In the meantime, though, these invasive species present opportunities for us to study their traces, learn more about their unseen behaviors, and compare these behaviors with their evolutionary histories. More science is always good, and in this respect, the Georgia barrier islands are the gifts that keep on giving.
 Traces of feral mammals on Sapleo Island: feral hog tracks strolling past a piece of feral cattle scat in a sandy road next to a maritime forest. What is the fate of these invasive species on the Georgia barrier islands, and how will these environments continue to change because of their presence? (Photo by Anthony Martin, taken on Sapelo Island.)
Traces of feral mammals on Sapleo Island: feral hog tracks strolling past a piece of feral cattle scat in a sandy road next to a maritime forest. What is the fate of these invasive species on the Georgia barrier islands, and how will these environments continue to change because of their presence? (Photo by Anthony Martin, taken on Sapelo Island.)
Further Reading
Ditchkoff, S.S., and West, B.C. 2007. Ecology and management of feral hogs. Human-Wildlife Conflicts, 1: 149-151.
Giuffra, E., Kijas, J.M.H., Amarger, V., Carlborg, Ö., Jeon, J.-T., and Andersson, L. 2000. The origin of the domestic pig: independent domestication and subsequent introgression. Genetics, 154: 1785-1791.
Mayor, J.J., Jr., and Brisbin, I.L. 2008. Wild Pigs in the United States: Their History, Comparative Morphology, and Current Status. University of Georgia Press, Athens, Georgia: 336 p.
Taylor, R.B., Hellgren, E.C., Gabor, T.M., and Ilse, L.M. 1998. Reproduction of feral pigs in southern Texas. Journal of Mammalogy, 79: 1325-1331.
Wood, G.W., and Roark, D.N. 1980. Food habits of feral hogs in coastal South Carolina. The Journal of Wildlife Management, 44: 506-511.
 We three burrows of Georgia coast are adorned with feces, showing that each of us is actively occupied by a ghost shrimp. In each burrow, the shrimp is probably just below the narrow aperture, doing a little housecleaning. (Photograph by Anthony Martin, taken on Jekyll Island, Georgia.)
We three burrows of Georgia coast are adorned with feces, showing that each of us is actively occupied by a ghost shrimp. In each burrow, the shrimp is probably just below the narrow aperture, doing a little housecleaning. (Photograph by Anthony Martin, taken on Jekyll Island, Georgia.)