It seemed all too fitting that author copies of my new book, The Evolution Underground: Burrows, Bunkers, and the Marvelous Subterranean World Beneath Our Feet (Pegasus Books, 2017) arrived on February 2. In the U.S., this is Groundhog Day, which is named after a burrowing animal and one in which its burrow plays a key role in its mythology. Did it cast a shadow or otherwise predict the weather for the next six weeks? No, but it may enlighten as you travel through geologic time, learning all about how animals and their burrows altered the world, and how animals used burrows to survive the worst the earth (or solar system) could toss at them.
 It’s here! After about two years from start to end, The Evolution Underground is out of its literary bunker and into your hands. (Photo by Anthony Martin.)
It’s here! After about two years from start to end, The Evolution Underground is out of its literary bunker and into your hands. (Photo by Anthony Martin.)
Is a book about burrows and burrowing animals too far beneath you to read? Well, as the immortal Kenny Loggins might say: Do what you like, and do it naturally.
The Evolution Underground is my seventh book and the second written overtly for a popular-science audience, following Dinosaurs Without Bones: Revealing Dinosaur Lives through Their Trace Fossils (2014, also by Pegasus Books). Dinosaurs Without Bones was a successful debut for me as a popular writer, with not-bad sales and mostly positive reviews (such as this, this, and this). That book was also my first attempt to make the word “ichnology” (the study of traces) more mainstream, and by using those always-charismatic dinosaurs as a hook. It worked, and I now think the percentage of people confusing ichnology with ichthyology has gone down ever so slightly since that book came out.
 It’s ichnology, not ichthyology. Make sure you get it right, because you do not want to be slapped by Batman.
It’s ichnology, not ichthyology. Make sure you get it right, because you do not want to be slapped by Batman.
For fans of Dinosaurs Without Bones, I’m happy to report my new book – which is officially published today, February 7, 2017 – includes dinosaurs and it’s about ichnology. But it also includes plenty of paleontology, geology, ecology, and good, old-fashioned natural history throughout. Moreover, this book gave me a chance to introduce readers to a panoply of animals representing the past 550 million years of earth history, while also exploring the big idea that burrowing impacted the evolution of many animals and their ecosystems.
 What’s it like to be a gopher tortoise? Kind of like being a subterranean landlord, considering that you might be sharing your burrow with 300-400 other species of animals.(Photograph by Anthony Martin, taken on St. Catherines Island, Georgia.)
What’s it like to be a gopher tortoise? Kind of like being a subterranean landlord, considering that you might be sharing your burrow with 300-400 other species of animals.(Photograph by Anthony Martin, taken on St. Catherines Island, Georgia.)
Along those lines, main themes of the book are expressed in subtitles I considered for it: How Burrows Changed the World and Better Surviving through Burrows. For the former, the mere collective action of burrowing animals – from the deep seafloor to mountaintops – is an essential part of how most ecosystems function. For the latter, burrows were all-natural bunkers enabling animals to escape the worst the Earth (or solar system) could throw at them and allowing their evolution to continue underground. Want to survive a mass extinction? Start digging.
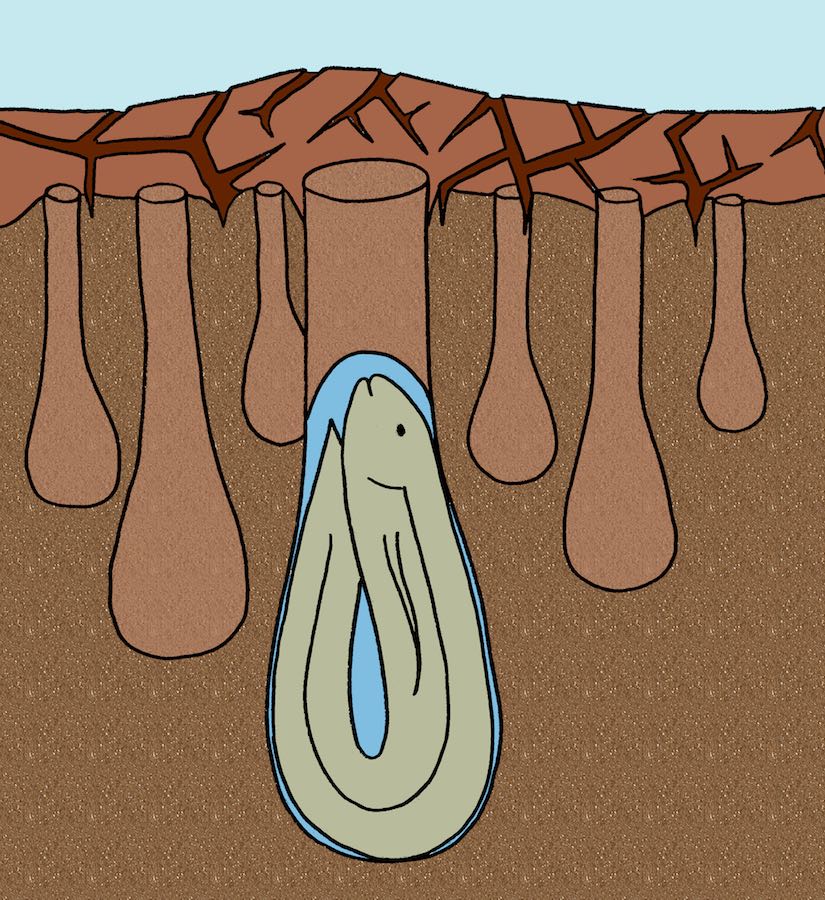 Lungfishes since the Devonian Period (more than 350 million years ago) have burrowed to avoid droughts, and their lineage has survived four mass extinctions. Coincidence? Probably not. (Original illustration by Anthony Martin, in The Evolution Underground (2017).)
Lungfishes since the Devonian Period (more than 350 million years ago) have burrowed to avoid droughts, and their lineage has survived four mass extinctions. Coincidence? Probably not. (Original illustration by Anthony Martin, in The Evolution Underground (2017).)
Must you buy this book, or at least persuade your local public library to get it? Well, yes, if you insist. Still, just in case you first need to know a bit more about the burrowing animals and geologic times represented in between its front and back covers, here’s a chapter list with brief descriptions of their contents. Thanks in advance, and I hope you and other readers enjoy reading it.
The Evolution Underground: Chapter Titles and Synopses
Chapter 1: The Wondrous World of Burrows – Did you know that alligators make burrows? They do indeed, and they’re awesome burrows. Learn how these body-armored saurians straight out of central casting from the Mesozoic Era provide superb living examples of how many animals use (or used) burrows to survive and thrive, thus symbolizing many of the main themes of the book.
Chapter 2: Beyond “Cavemen”: A Brief History of Humans Underground – Since the time of living in caves, humans have gone beneath the Earth’s surface during times of environmental or societal stress, and we still do. In this chapter, travel to Turkey, China, Russia, Australia, Canada, and the far-off exotic land of Pennsylvania (home of weather-predicting groundhogs) to marvel at how humans, time and time again, have looked below when seeking safety.
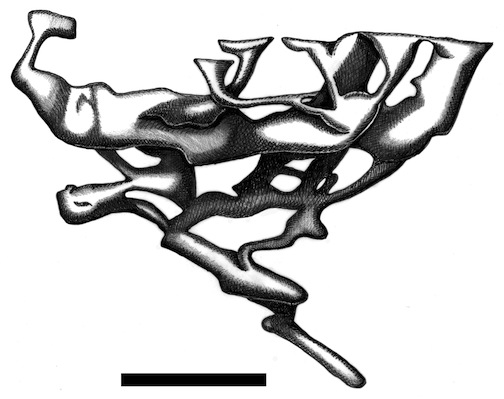
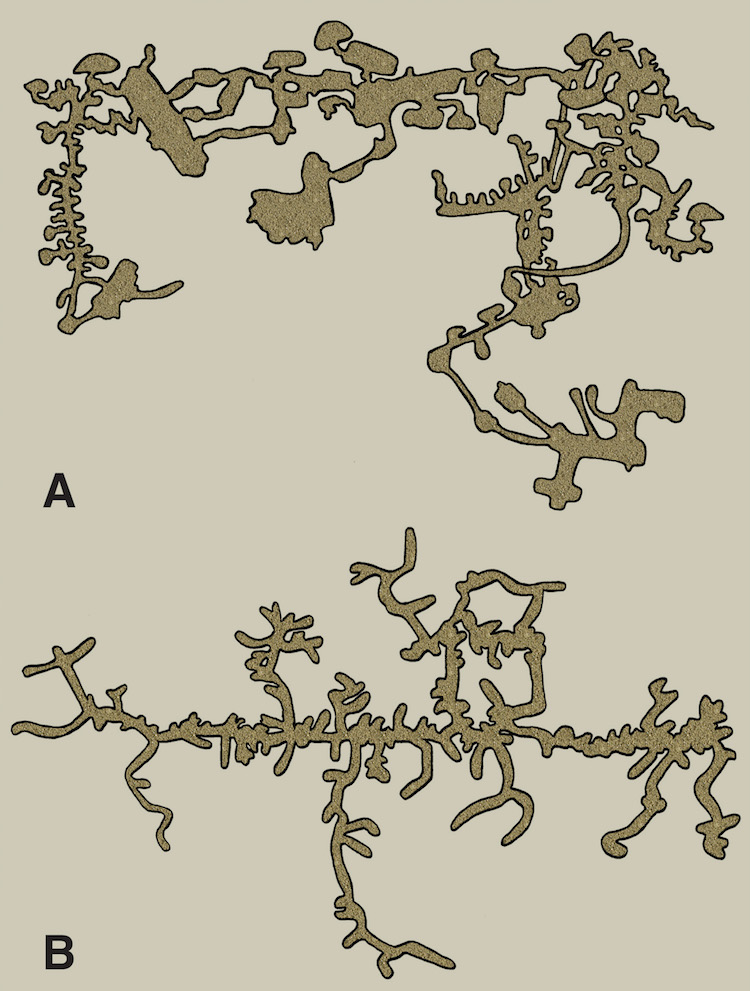 One of these is a map of a naked mole rat burrow system, and the other is of an underground city made by humans in central Turkey. Which is which? That might be one of many questions answered by reading my new book. (Original illustration by Anthony Martin, in The Evolution Underground.)
One of these is a map of a naked mole rat burrow system, and the other is of an underground city made by humans in central Turkey. Which is which? That might be one of many questions answered by reading my new book. (Original illustration by Anthony Martin, in The Evolution Underground.)
Chapter 3: Kaleidoscopes of Dug-Out Diversity – Gopher tortoises of the southeastern U.S. dig burrows that are both deep and meaningful, as these burrows host underground menageries of many other species, boosting the biodiversity of their ecosystems. How did tortoises and other turtles evolve and survive mass extinctions of the past? If you answered “burrowing,” you’re catching on to what this book is about.
Chapter 4: Hadean Dinosaurs and Birds Underfoot – Although burrowing dinosaurs of the Mesozoic past were apparently rare, a few of their living descendants (birds) evolved to put their nests not in trees, but underground. In this chapter, penguins, puffins, shearwaters, owls, kiwis, bee-eaters, and other birds raising underground families are lauded for their digging family values.
Chapter 5: Bomb Shelters of the Phanerozoic – This chapter opens with a piece of fiction about a Lystrosaurus (or two) embarking on a post-apocalyptic journey. This allegory conveys how burrowing helped their kind and a few other animals to survive the worst mass extinction in the history of life at the end of the Permian Period (about 250 million years ago). This chapter also summarizes other mass extinctions and how burrowing provided an advantage for making it through the worst ecological crises of the geologic past.
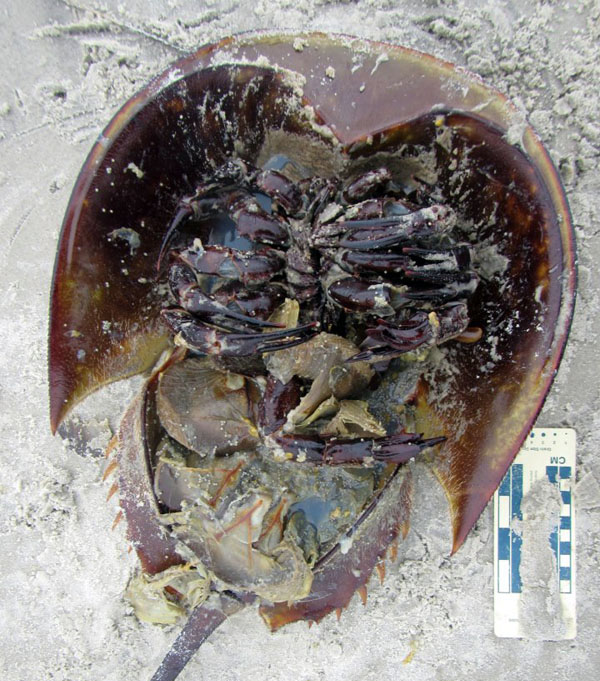
Chapter 6: Terraforming a Planet, One Hole at a Time – When did animals move from the sea to freshwater and then onto land? Burrowing may have helped animals to make transitions from such environmental extremes, which ultimately resulted in their shaping landscapes as we know them today. Featured animals in this chapter include trilobites, horseshoe crabs, lungfish, amphibians (frogs, toads, salamanders), lizards, and snakes.
Chapter 7: Playing Hide and Seek for Keeps – For a long time, all animal life was superficial, living either on seafloor surfaces or just underneath. Then about 550-540 million years ago, animals starting plumbing deeper. What caused this downward shift, and how did animals’ churning of oceanic sands and muds forever change the oceans, atmosphere, and the evolution of life? Also, the evolution of predators gave animals yet another reason to burrow: That is, before the predators started burrowing, too, starting an underground “arms race” that continues through today.
Chapter 8: Rulers of the Underworld – What animals are the real ecosystem engineers for our planet? Mostly the small and spineless ones, invertebrates. This chapter starts with those marvelous earthworms that so beguiled Charles Darwin, then pays tribute to the amazing feats of burrowing and animal architectures created by ants, crayfish, crabs, lobsters, and more.
Chapter 9: Viva La Evolución: Change Comes from Within – This chapter starts with the second fictional story in the book, following the exploits of an ecological hero – a pocket gopher – following the 1980 volcanic eruption of Mount St. Helens. The rest of the concluding chapter of The Evolution Underground looks at burrowing mammals (especially rodents), but also considers the largest burrowing animals of all time. Also, what can we as mammals learn from our fellow furry underground relatives as we head into an uncertain future posed by rapid climate change?
Appendix: Genera and Species Mentioned in The Evolution Underground – A listing of the animals name-dropped in the book, some of which may surprise you.
 What are you waiting for? Leave your underground hidey-hole and get my book! P.S. Thanks for reading it. (Photograph by Anthony Martin, taken in Decatur, Georgia.)
What are you waiting for? Leave your underground hidey-hole and get my book! P.S. Thanks for reading it. (Photograph by Anthony Martin, taken in Decatur, Georgia.)